Tuesday, 9th June 2020
Hi!
This is your planning for today:
1) Do the kahoot of unit 5.
2) Visit the Social Science page on the blog. Let's deal with the last unit!
Monday, 8th June 2020
Good morning!
This is your planning for today:
1) Do the kahoot about unit 5. It will be available up to Wednesday 10th.
Click on the following link:
2) Tomorrow, we will continue with the last unit of Social Science.
We will not have the time to study unit 6 of Natural. It 's about new technologies, computers and the Internet.
As you can see, they are contents quite familiar to you.
If you have the time, have a look to the unit on your Natural Science digital book, following these steps:
Thank you!
Friday, 5th June 2020
Hi guys!
This is your planning for today:
1) Check your homework. Thse are the answers:
ACTIVITY 1
a) The link between electricity and
magnetism was discovered by Oersted.
b) TRUE
c) Electromagnets can be switched off.
d) The north pole of two bar magnets
repel each other.
e) TRUE
f) TRUE
ACTIVITY 2
ACTIVITY 6
a) The north pole of a
permanent magnet attracts the
pole of another permanent magnet.
b) Opposite poles , whereas alike poles .
c) The magnetosphere is
the around the .
d) The magnetosphere protects
us from .
e) Magnets attract magnetic
materials, for example .
Activity 7
Activity 8
2) Study the unit. On Monday I will post the kahoot.
If you have any question, please, ask me by email.
Have a nice weekend!
Thursday, 4th June 2020
Good morning!
This is your planning for today:
1) Continue with the homework that I post yesterday.
2) Study the unit. On Monday I will post the kahoot of the unit.
Thank you!
Wednesday, 3rd June 2020
Hi!
This is your planning for today and tomorrow (you have 2 days to do all this homework, up to Friday):
1) Revise the unit doing the following exercises:
Hi!
This is your planning for today and tomorrow (you have 2 days to do all this homework, up to Friday):
1) Revise the unit doing the following exercises:
Next week, you will have to take the kahoot of this unit.
If you have any question, please, ask me by email.
Thank you.
If you have any question, please, ask me by email.
Thank you.
Tuesday, 2nd June 2020
Good morning!
This is your planning for today:
1) Check your homework. These are the asnwers:
2) Let's continue learning about electromagnets.
Electromagnetism is used in many different gadgets in our dialy life.
Electromagnets are made of:
- Wire coiled (cable enroscado)
- Iron rod (barra de hierro)
- Battery
Electricity flows through the circuit, producing a magnetic field.
The magnetic field is only produced when electricity is flowing through the circuit.
Here you have examples of gadgets that work using an electromagnet:
maglev = a kind of train
Watch this video to check how a hand-made bell works using an electromagnet. If you want to, try this experiemnt at home, always with an adult's help and supervision.
3) Put these contents into practice doing the following activities:
Activity book, page 9 exercise 3
Activity book, page 13 exercise 10 and 11
If you have any question, please, ask me by email.
Thank you.
Monday, 1st June 2020
Hi!
This is your planning for today:
1) Check your homework. These are the answers:
2) Today let's learn about electromagnetism. It is on page 92 and 93 on your Natural Science digital book.
Electromagnetism: it studies how electricity and magnetism are related and work together.
There have been different scientists that have studied this relation:
Howelectricity and magnetism are related?
1) Electricity produces a magnetic field when it flows through a wire (cable).
2) A magnet creates electricity: if we move a magnet near a coil of wire (un muelle de cable) it will create a magnetic fild. As a result, electrons will move from the orbit of one atom, to the orbit of another atom. In this way, electricity is created.
Click on this video to see how a power station works. In this case, this station uses the force of water when it moves.
3) Practice these contents doing:
Exercise 2 on page 93
Activity book, page 9 exercise 1 (the one you have on paper)
If you have any question, ask me by email.
Thank you.
Friday, 29th May 2020
Good morning everyone!
This is your planning for today:
1) Revise what we have learnt up to this moment about electricity and magnetism.
Watch this video about how magnets work.
2) Let's continue learning about the Earth magnetic field. It's on page 90 and 91 on your Natural Science digital book.
Magnetosphere: Earth's magnetic field. It protects life on the Earth from solar radiation.
The Earth would have a bar magnet (imán) in its centre.
IMPORTANT: it's not the same the north Pole on planet Earth, and the north pole of the magnetosphere.
They are just the opposite:
North Pole on planet Earth __ Magnetosphere's south pole.
The centre of planet Earth is made of liquid iron.
This is the reason why the Earth rotates slowly on its axis (rotation movement).
Rotation causes the magnetic field.
Watch this video to make this explanation clearer:
Why theEarth's magnetic field is useful for us?
1) When we use a compass (brújula): a compass has a magnetised needle (aguja magnética). The north pole of the needle will point at the North Pole of the Earth (south pole of the magnetosphere).
2) The Earth's magnetic field protects us from solar radiation that will damage life on the Earth.
3) Practice doing the following exercises:
- Exercise 2 on page 91 on the digital book.
- Activity book (the one you have on paper), page 11, exercises 6, 7 and 8.
If you have any question, please, ask me by email.
Have a nice weekend!
Thursday 28th May 2020
Hi!
This is your planning for today:
1) Check your homework. These are the answers:
A) BAR MAGNETS CAN´T BE SWITCHED ON AND OFF.
B) IRON FILING CAN BE USED TO SHOW THE MAGNETIC FIELD.
C) A DIAGRAM OF A MAGNETIC FIELD HAS LINES.
D) WHEN TWO MAGNETS ATTRACT EACH OTHER, THE LINES ARE CURVED FROM ONE MAGNET TO THE OTHER.
E) IN A MAGNETIC SUBSTANCE, THE ELECTRONS MOVE FROM THE ORBIT OF ONE ATOM TO ANOTHER ONE.
F) WE CAN IDENTIFY A MAGNET BY TESTING IF IT IS REPELLED BY ANOTHER MAGNET.
2) Revise what we have learnt yesterday about magnetism. Here you have a revision:
Magnetism is an invisible force between to magnetic objects or one magnetic object and materials like iron.
Magnetism is produced in the same way as electricity: when electrons move from the orbit of one atom to the orbit of another atom.
Magnetic bars (imanes) are magnetically charged permanently.
They have two poles:
- North pole: positive charge (+)
- South pole: negative charge (-)
Opposite poles (north pole + south pole): atraction
The same pole (north + north // south + south): repulsion
3) Revise those contents, doing exercises 4 and 5 on page 10 on your activity book (the one you have on paper).
Thank you.
Wednesday, 27th May 2020
Good morning!
This is your planning for today:
1) Revise what we have learnt yesterday about electric circuits.
2) Today, let's learn about magnetism.
3) Practice this information doing exercise 2.
Thank you!
Tuesday, 26th May 2020
Hi!
This is your planning for today:
1) Revise what we have learnt yesterday about electricity.
2) Let's continue learning about electricity, especially about simple electric circuits.
The components in a circuit are:
- Battery: where electricity is stored.
- Two terminals, in this case, wires (cables): they are made of metal, cooper (cobre). It is a good conductor of electricity.
You can do this experiment at home with and adult (only with an adult). If you try it, please, share your experience with me by email 😊
3) Do the following activities:
Digital book page 87. Exercise 2.
If you have any questions, ask me by email please.
Thank you.
Monday, 25th May 2020
Good morning!
This is your planning for today:
1) Open your Natural science digital book on page 86 and 87. Read the information about electric charges.
- Everything in the universe is made of atoms.
- Atoms are made of:
When atoms lose electrons (-), matter becomes postively charged (+).
When atoms lose protons (+), matter becomes negatively charged (-).
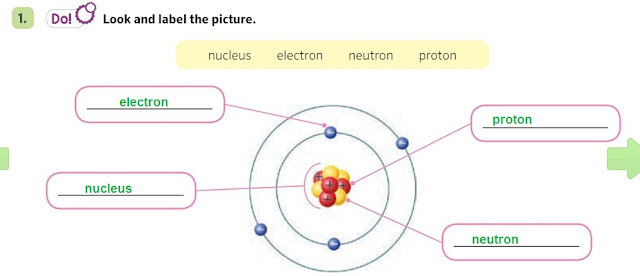
- There are 2 main parts in an atom: the nucleus and the orbits.
- The nucleus is made of protons and neutrons
- Electrons rotate around the nucleus in its orbits.
- How electricity is produced?
Positive charged objects attract negative charged objects
( + attract - )
Negative charged objects attract negative charged objects
( - attract + )
2) Open your Natural Science activity book on page 23. Do exercise 1.
Thank you.
Friday, 22nd May 2020
Hi again!
Let's start unit 5: Electricity and magnetism.
1) Do the cover on your Natural Science notebook.
2) Read the information that presents you the unit.
2. Do exercise 1 a) in a chart (tabla).
Have a nice weekend!
Friday, 8th May 2020
Good morning!
Today we are starting a new unit of social science, so, let's go back to the "Social Science" page on this blog.
Thursday, 7th May 2020
Good morning!
This is your planning for today:
1) Take the kahoot about unit 4, matter. Remember the rules:
- You should complete it before Monday, 11th 12:00 PM.
- Do it when you are ready.
- Do it only once (una sola vez), unless you have problems with the Internet. In this case you can do it again writing your name and number 2. For example: "MARINA 2"
- Write your real name. Otherwise, you won't have any mark.
Thank you.
Wednesday, 6th May 2020
Good morning!
This is your planning for today:
1) Check your homework. These are the answers:
Exercise 3. How would you separate a mixture of sugar and rice? Draw a picture and write asentence for each step of the process.
To separate a mixture of sugar and rice we need to methods: first, filtration. Then, evaporation.
1 Add water to the mixture to dissolve sugar (soluble solid).
2 Filter the mixture to separate the soluble solid from the insoluble one. FILTRATION
3 Evaporate the remaining solution to separate the sugar from the water. EVAPORATION
2) Study all the unit and all the exercises that you have done to take the kahoot that I will post tomorrow.
Remember, the more you study, the better the result will be.
Thank you!
Tuesday, 5th May 2020
Hey!
This is your planning for today:
1) Study for your kahoot that you will have to do on Thursday.
2) Check your homework. These are the answers:
3) Continue revising the unit doing the following exercises:
Thank you.
Monday, 4th May 2020
Hi guys!
This is your planning for today:
1) Study all the unit. On Thursday, you will have to take a kahoot about it. If you have any question, please, ask me by e-mail.
2) Check your homework. These are the answers:
Activity book, page 8. Exercise 12.
Digital book page 80. Exercise 1.
a) reversible / non reversible
b) colour / texture / colurs / textures
c) homogeneous / evaporation
d) separation / condensation / separation /dissolving / insoluble
e) oxygen / carbon dioxide
f) sugars / alcohol / yeast / bacteria
g) electrons / oxygen atoms / air
Digital book page 80. Exercise 2.
a) What three substances combine in this chemical reaction?
In this chemical reaction, wood, oxygen and heat are combined.
b) What substances are produced?
A combustion like this produces: ashes (cenizas), smoke (humo), carbon dioxide.
Digital book page 80. Exercise 5
Digital book page 80. Exercise 6
Digital book page 80. Exercise 7
a) A reducing agent is a substance that gains electrons during the process of fermentation.
A reducing agent is a substance that gains electrons that loses electrons during the process of oxidantion.
b) A solution is a mixture of two solids in which both components are easy to see.A solution is a mixture of one or more liquids with one or more other susbtances in which both components can't be distinguished.
c) Chemical changes are reactions between two substances that are easy to reverse.
Chemical changes are reactions between two or more substances that are irreversible.
d) We make cheese, yogurt and kefir by oxidation.
We make cheese, yogurt and kefir by fermentation.
(kefir is something similiar to yogurt)
Digital book page 80. Exercise 8
a) Cider, because it is the only one where yeast consumes sugar and turns it into alcohol.
b) Seawater, because it is an homogeneous mixture. The rest are heterogeneous mixture.
(concrete = hormigón)
c) Filtration, because it is a process to separate mixtures. The rest are chemical changes.
d) Solute, because the rest are processes to separate mixtures.
3) Revise the unit doing these exercises on your Natural Science notebook.
If you have any question, please, ask me by email.
Thank you.
Thursday, 30th April 2020
Good morning!
This is your planning for today:
1) Study all the unit. Next week you will have to take a kahoot about it. If you have any question, please, ask me by e-mail.
2) In order to put into practice what you have learnt in this unit, do the following exercises:
- Natural Science digital book. Page 80 exercises 1 and 2.
- Natural Science digital book. Page 81 exercises 5, 6, 7 and 8.
Have a nice weekend! 😊
Wednesday, 29th April 2020
Hi everyone!
This is your planning for today:
1) Check your homework. These are the answers:
Activity book. Page 8 exercise 11.
Book. Page 77 exercise 2.
a) We can make cheese and beer using yeast.
We can make bread, cider and beer using yeast.
b) Yeast is a multicellular plant. Yeast is a inucellular fungus.
c) Yeast produces oxygen and sugar when it carries our nutrition.
Yeast consumes sugar and it converts it into alcohol and carbon dioxide.
d) Fermentation is a chemical reaction that needs oxygen. Fermentation is a chemical reaction that needs yeast or bacteria.
2) Revise what we have learnt about chemical changes. Then do exercises 12 and 13 on page 8 on your activity book.
3) Study all the unit. From tomorrow on, we will revise it and next week you will have to take a kahoot about it.
If you have any question, please ask me by email.
Tuesday, 28th April 2020
Hey!
This is your planning for today:
1) Let's continue learning about chemical changes. You have learnt about combustion and oxidation. Today you will learn about fermentation.
Open your Natural Science digital book on page 76 and 77. Follow these instructions:
Fermentation is a chemical change used mainly to treat food. In fermentation bacteria, yeast (hongos) an other microorganisms are used.
In fermentation enzymes (yeast) take in sugar and they change it into alcohol and CO2.
Fermentation is used to make food. For example:
Cider (sidra): It's a type of an alcoholic drink made of apples. Apples contain sugar. Yeast consumes this sugar and it turns it into alcohol and CO2.
CO2 is the bubbles (burbujas) that you can see in this kind of drinks.


Bread: in order to make bread, you know flour and water are needed. Then, you also have to add yeast (la levadura es un tipo de hongo) to the dough (masa).
Yeast consumes de starch (almidón) in flour, producing CO2.
CO2 expands bread and produces bubbles in the dough.


Cheese: in order to make cheese we need milk. We need to add enzymes (bacteria) to become milk solid.
Once it is solid, it is pressed and salt is added. It is preserved during months in a cared atmosphere to obtain the cheese we finally consume.








2) Now, do activity 2 on page 77 on your Natural Science notebook.
Fermentation is the most difficult change of the three chemical changes that you have studied. If you have any question, please ask me by e-mail.
Thank you.
Monday, 27th April 2020
Good morning!
This is your planning for today:
1) Check your homework. These are the answers:
Activity book on page 7 and do exercises 8, 9 and 10.
Exercise 2 on page 73
2) Revise what you have learnt on Friday about oxidation. Then, do exercise number 11 on page 8 on your activity book.
Thank you.
Friday, 24th April 2020
Good morning guys!
This is your planning for today:
1) Today you will continue learning about chemical changes, specially about oxidation.
It is on page 72 and 73 on your Natural Science digital book.

Oxidation is a chemical reaction. In oxidation, the substance changes its appearance and it loses some of its properties.
Remember: matter was made of atoms. Each atom is made of:
electrons + protons + neutrons
In oxidation, one susbtance loses electrons. They are transferred to another substance.
- The substance that loses electrons is called reducing agent (it reduces its amount of electrons)
- The substance that gains electrons is called oxidising agent (oxidising comes from OXYGEN)
As you can see, there are 2 types of oxidation:
1) Rusting: it is the chemical reaction suffered by some metals. Oxidation takes place when metal is exposed to oxygen and moisture (water).
- The metal is the reducing agent: it loses electrons.
- Oxygen is the oxidising agent: it gains electrons.
When a metal is oxidated, its properties and characteristics change. For example, its colour: it turns orange.
2) Browning: this chemical reaction should be familiar for you. It usually happens when fruits and vegetables are exposed to oxygen.
- Fruit is the reducing agent (it loses alectrons)
- Oxygen is the oxidising agent: it gains electrons.
When fruits suffer oxidation, their properties and characteristics changes. They turn brown (that's why the process is called BROWNING) and sometimes it is not recommended to eat them anymore.
Have a nice weekend! 😊
Thursday, 23rd April 2020
Hi guys!
This is your planning for today:
1) Check your homework. These are the answers:
2) Practice what you have learnt yesterday. Open your Natural Science activity book on page 7 and do exercises 8, 9 and 10.
Thank you!
Wednesday, 22nd April 2020
Hi everyone!
This is your planning for today:
1) Check your homework. These are the answers:
Book, page 69. Exercise 2
Activity book, page 6. Exercise 6
Activity book, page 6. Exercise 7
2) Open your Natural Science digital book. This is the sequence you have to follow (you asked me yesterday):
Today we will cotinue learning about chemical changes, specially, about combustion.
But first, do you remember the difference between physical and chemical changes?
⇩
Irreversible = after a chemical change, the substance cannot return to its original form.
For example: if we burn a piece of paper, it will be burnt forever.
That's why combustion is one type of chemical changes.
To burn a piece of paper we need:
1) Paper = fuel
2) Fire = heat
3) Oxygen
paper + fire + oxygen = combustion
If we eliminate one of these elements, it will stop burning. That's why it is so important to close the windows in case of fire, to eliminate oxygen from the room.
You can try this experiment at home, ALWAYS WITH AN ADULT NEXT TO YOU. Water is not essential.
When we burn paper or wood, we also obtain thermal energy (calor). In science this is called exothermic reaction.
The main disadvantage of combustion is the pollution (CO2) that it produces.
The consequences are:
1) Global warming: the temperature of the planet increases. As a results, the sea level increases, too.
2) Respiratory diseases (enfermedades)
3) "Acid rain": rainwater + sulphur dioxide (toxic gas)
3) On your notebook, do activity 2 on page 71 on your Natural Science digital book.
Thank you!
Tuesday, 21st April 2020
This is your planning for today:
1) Revise what we have studied yesterday about how to separate mixtures.
2) Then, open your activity book on page 6 and do exercises 6 and 7.
Thank you!
Monday, 20th April 2020
Hello!
This is your planning for today:
1) Do the kahoot I posted on Friday:
2) Today, we are going to continue studying mixtures. We are on page 68 and 69 on your digital book (register on blinklearning if you haven't done it yet).
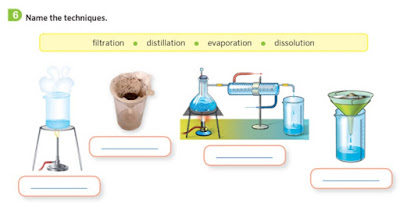
There are 3 different techniques to separate the different components in mixture:
- Filtration: this tecnique is used to separate water and sand. Try to mix sand and water. You can't. Sand is an insoluble solid (it can't be mixed with a liquid). That's why this mixture passes through a filter, sand will remain on the filter. Only water will pass.
- Evaporation: this technique is used to separate soluble solids (solid that you can mix with water, for example, sugar, salt) from a liquid. The mixture is heated. Water will be evaporated, and the solid will remain.
- Destilation:this technique is used to separate 2 liquids. The mixture is heated. The liquid with the lowest boiling point will be evaporated first. It will turn into a gas.
3) Try these experiments at home if you have the materials. You can try the experiment on page 69, too.
4) Then, do activity 2 on page 69 on your Natural Science notebook.
Thank you.
Friday, 17th April 2020
Hi everyone!
This is your planning for today:
1) Check your homework. These are the answers:
Book, exercise 2.
Activity book, page 5. Exercise 4.
2) Revise what we have learn in this unit up to this moment doing this kahoot:
- Don't do the kahoot if you haven't done the activities I have sent you during the week. The more you study, the better results you will obtain in the kahoot.
- Write your real name and, if it is necessary, your surname.
- Do the kahoot only once (1). You can only do it twice (2) if you have problems with the Internet or the app. If you need to do it twice, write your name and number 2 (for example, Marina 2).
- This kahoot will be available up to next Tuesday, 21st April.
Have a nice weekend! See you on Monday 😊
Thursday, 16th April 2020
Good morning guys!
This is your planning for today:
1) Check your homework. These are the answers:
2) Let's continue learning about mixtures and solutions. But, what's a solution? Pay attention to this video:
A solution is a mixture of 1 or more liquids + other substances.
For example: try to mix milk and colaCao, water and sugar, milk and sugar. They are all solutions.
A solution is a homogeneous mixture, because you cannot distinguish the different components.
Let's focus on the example of colaCao: this time, we will mix: milk + chocolate + sugar = colaCao
Milk = solvent (It's the liquid)
Chocolate = solute (component)
Sugar = solute (component)
ColaCao = solution (the mixture)
3) Now, practice these concepts doing the following exercises:
IMPORTANT: Revise these concepts. Tomorrow I will leave a kahoot to check what we have learnt up to this moment. The more you revise and study, the better the result will be.
Thank you.
Wednesday, 15th April 2020
Hi!
This is your planning for today:
1) Check your homework. These are the answers:
2) Today we are going to learn about pure substances (sustancias puras) and mixtures (mezclas).
Do you remember what's O2? Oxygen.
Oxygen is a pure substance because it is only made of 1 component: oxygen.
But, remember HO2 was water, because water is made of hydrogen + oxygen.
It's a compound (compuesto). Water is made of 2 elements: hydrogen and oxygen.
The difference between a pure substance and a mixture is that:
- You cannot separate the different elements in a pure substance.
- But you can separate the different elements in mixtures.
1. Homogeneous mixtures (mezclas homogéneas): in this kind of mixtures, you cannot see the different elements in it.
For example, look at water. We said water is made of hydrogen + oxygen. Can you distinguish these 2 elements in water? NO.
Another easy example that you can check at home: colaCao. You mix milk + chocolate. Once it is mixed, you can distinguish chocolate and milk.
2. Heterogeneous mixtures (mezclas heterogéneas): in this kind of mixtures, you can see the different elements in it.
For example, look at a salad.

You can see the different elements: lettuce + tomato + carrot
3) Time to practice. Natural Science activity book, page 5, only exercise number 5.
If you have doubts, check these mixtures doing the experiment at home.
IMPORTANT: Remember you must register in www.blinklearning.com to access to your Science digital books.
Thank you!
Tuesday, 14th April 2020
Good morning guys! We're back again. How was your Easter break at home?
This is your planning for today:
1) Check your homework. These are the answers.
Book, exercise 2.

a) FALSE. REFLECTION OCCURS WHEN LIGHT HITS A SOLID SURFACE.
b) FALSE. LIQUIDS USUALLY HAVE A SMOOTH SURFACE.
c) TRUE
d) TRUE
Activity book page 4, exercise 3.

a) Light is a form of ENERGY that travels in WAVES.
b) Electricity is a FORM OF ENERGY that is created when ELECTRONS MOVE FROM THE ORBIT OF ONE ATOM TO THE ORBIT OF ANOTHER.
2) Revise the contents of unit 4 that we have studied before holidays. Check all the information on the blog again: pictures, vídeos, my personal comments...
3) Then, do the following activities on your Natural Science notebook. Copy the date and you must copy the instructions (enunciados).
1. Circle the correct answer.
1. What is light?
a) Light is a form of matter. b) Light is a form of energy.
2. How does light travel?
a) Light travels in hoops. b) Light travels in waves.
3. What state of matter usually has a smooth surface?
a) Liquid matter b) Gaseous matter
4. What state of matter usually has a rougher surface?
a) Solid matter b) Liquid matter
5. What state of matter usually produces a reflection?
a) Gaseous matter b) Liquid matter
2. Complete the definitions.
a) Reflection occurs when _____________ hits an object with a _____________ surface. The light is reflected ______________ and we see a reflection on the surface of the object.
b) Diffusion occurs when ______________ hits an object with a _______________ surface. The light is reflected ______________ and we don’t see a reflection on the surface of the object.
3. Answer the questions.
a) What two things is the nucleus of an atom made up of? _______________________________________________________________
b) What do we call the movement of electrons around the nucleus? _______________________________________________________________
c) Which part of an atom moves to another atom in order to produce electricity? _______________________________________________________________
d) What electrical charge does matter have when it loses electrons? _______________________________________________________________
If you have any question, please, ask me by e-mail.
See you!
Thursday, 2nd April 2020
Hi guys!
This is your planning for today:
1) Check your homework. These are the answers.
Activity book page 4, exercise 1.

Picture 1:
REFLECTION
When light hits a SMOOTH surface, it's reflected IN A REGULAR WAY.
Picture 2:
DIFFUSION
when light hits a ROUGH surface, it's reflected IN AN IRREGULAR WAY.
Activity book page 4, exercise 2

You should draw straight lines, because a mirror reflect light in a regular way.
Objects with reflective surfaces: MIRROR, LIQUID WATER
2) Today we will learn about electricity.
- Everything in the universe is made of atoms.
- Atoms are made of:
When atoms lose electrons (-), matter becomes postively charged (+).
When atoms lose protons (+), matter becomes negatively charged (-).
- There are 2 main parts in an atom: the nucleus and the orbits.
- The nucleus is made of protons and neutrons
- Electrons rotate around the nucleus in its orbits.
- How electricity is produced?
Positive charged objects attract negative charged objects
( + attract - )
Negative charged objects attract negative charged objects
( - attract + )
3) Now, do the following exercises:
- Book, exercise 2. Here you have the picture of the exercise. Do it on your notebook, please.
- Activity book page 4, exercise 3.
This Easter break you won't have extra homework. Use this time to update (poneros al día) to all the work we have done during this period without classes at school.
Remeber: that period is not a break (holidays). We are working in a different way and all this work is compulsory (obligatorio) for you.
Enjoy your holidays at home! See you the next 14th April on this blog 😊
Wednesday, 1st April 2020
Hey!
This is your planning for today:
1) Check your homework. These are the answers:

Exercise 1
a) PICTURE A SHOWS A PHYSICAL CHANGE
PICTURE B SHOWS A CHEMICAL CHANGE
PICTURE C SHOWS A CHEMICAL CHANGE
PICTURE D SHOWS A PHYSICAL CHANGE
b) CHEMICAL CHANGES PRODUCE NEW SUBSTANCES.
c) PRECIPITATION CHANGES THE STATE OF WATER VAPOUR (GAS) INTO LIQUID (RAIN) OR SOLID (SNOW).
d) WHEN WE FRY AN EGG, A CHEMICAL CHANGE IS PRODUCED.
Exercise 2
2) Today we are going to learn about light and how light is reflected.
- Reflection is a natural phenomenon. That happens when light rays hit on a surface (matter) and it changes its direction.
- States of matter: we can find matter in three different states: solid, liquid or gas.
- Gas matter: it doesn't reflect light.
- Liquid matter: it reflects light.
- Solid matter: it depends on the surface, it can reflect matter in a regular or and irregular way.
- There are two ways in which light is reflected:
- Diffusion: light is reflected on a rough (rugosa) surface in an irregular way.
3) Now, do this two exercises from the activity. You have your Natural Science activity book. It's page 4, exercises 1 and 2.
Just in case you don't have it, I'll attach the pictures:
If you have any question, just ask me by e-mail.
Thank you!
Tuesday, 31st March 2020
Hello!
This is your planning for today:
1) Do the cover of unit 4 "Matter" if you haven't done it yet.
2) Read the text on this picture. It is about physical and chemical changes. Here I post and explanation and a video with examples to help you.
3) Now, do exercises 1 and 2. If it's possible try these changes at home (ONLY IF IT'S POSSIBLE; DON'T BURN ANYTHING PLEASE) to check how matter changes.
Thank you!
Monday, 30th March 2020
Good morning everyone!
This is the Natural Science's page. Here you will find all the lessons about Natural Science.
I know you don't have your books. Don't worry. I will post it here and apart from that, I'm trying to obtain an online book for you (I'm speaking with Oxford).
Today, you only have to do the cover on your Natural Science notebook. It's unit 4 and the title is "Matter".
Thank you!


















































































































































Profe perdón pero me salí en la primera pregunta 😅
ResponderEliminarHola Mae,
EliminarVuélvelo a intentar como MAE 2.
Un saludo.
Marina
hola buenas tardes soy said de 6A profe donde pone 17 de abril el ejercicio 4 que mandaste para corregir en la ultima frase no hay nada que hay que poner?
ResponderEliminarHola Said,
ResponderEliminarNo me di cuenta de que no salía. Antes de decirte la respuesta, ¿cuál crees que es la palabra que falta ahí?
Hola soy Rodrigo. Estoy en Segovia y no puedo coger el libro digital de naturales
ResponderEliminarHola Rodrigo,
EliminarDiscúlpame, pero acabo de ver tu comentario en el blog. La próxima vez escríbeme mejor al correo con este tipo de preguntas.
El libro digital lo tienes en la cuenta de blink, a la que tienes acceso desde cualquier dispositivo. De todos modos, siempre suelo poner las imágenes del contenido por si alguien tiene algún problema para acceder.
Pese a este problemilla, me consta que estás siguiendo bien el trabajo.
Un saludo y te pido disculpas de nuevo.
Este comentario ha sido eliminado por el autor.
ResponderEliminar